Our Research
Helminth regulation of intestinal stem cell fate and function
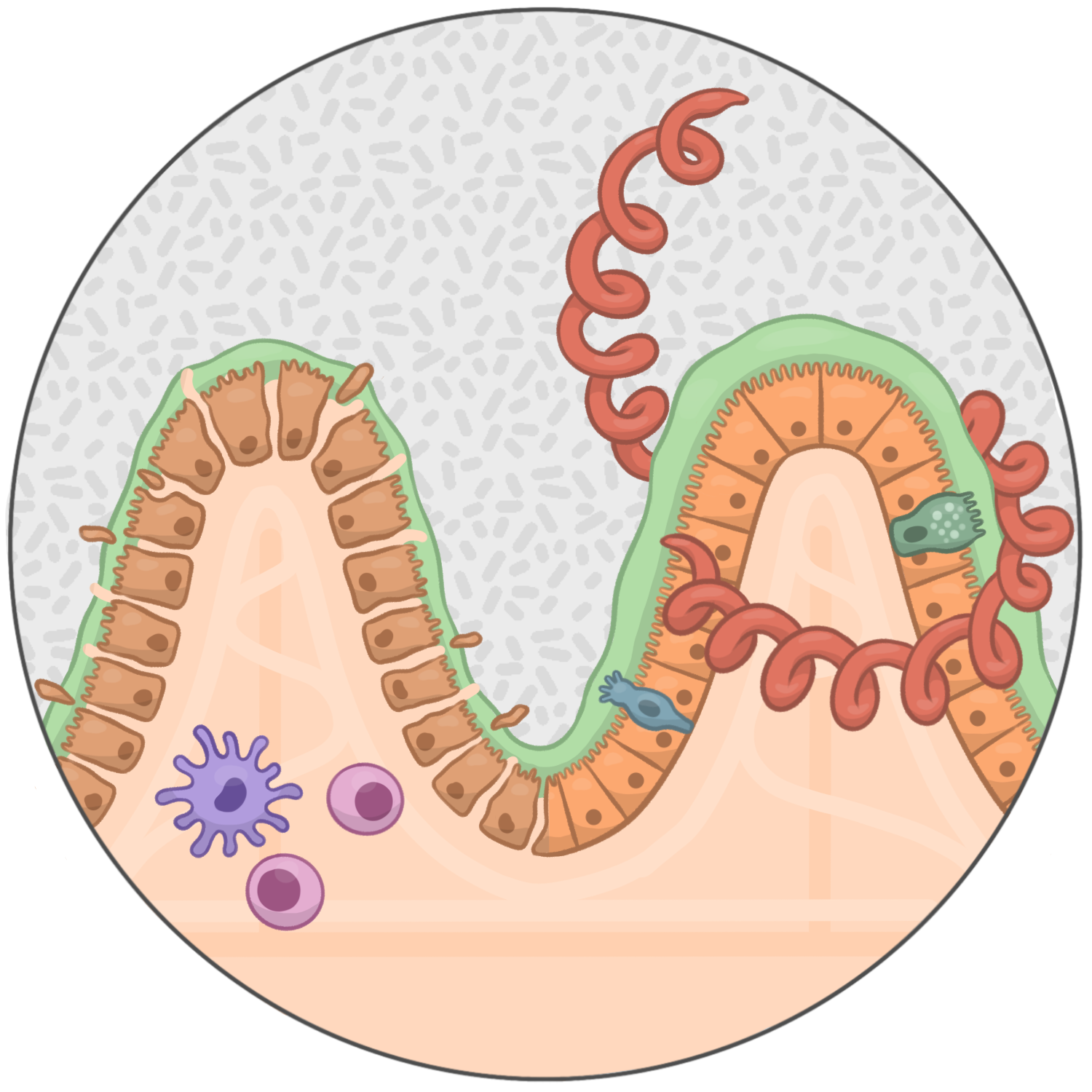
As frontline effectors of barrier integrity, a key attribute of the intestinal stem cell (ISC) compartment is its ability to undergo extensive transcriptional reprogramming in response to injury to promote rapid regeneration of the tissue and maintain barrier integrity.
We have previously demonstrated how helminths directly target the ISC compartment to revert the epithelium into a highly regenerative, yet immature fetal-like state. This fetal-like reversion coincides with inhibition of cytokine-driven differentiation of secretory cell lineages that promote parasite expulsion. These results highlight how helminths directly compete with their hosts for control of the ISC compartment to promote chronic infection.
Our lab develops and applies novel discovery based and hypothesis-driven approaches to identify helminth-derived molecules that drive ISC reprogramming and investigates how this impinges on host defense against infection.
Helminth regulation of epithelial immunity
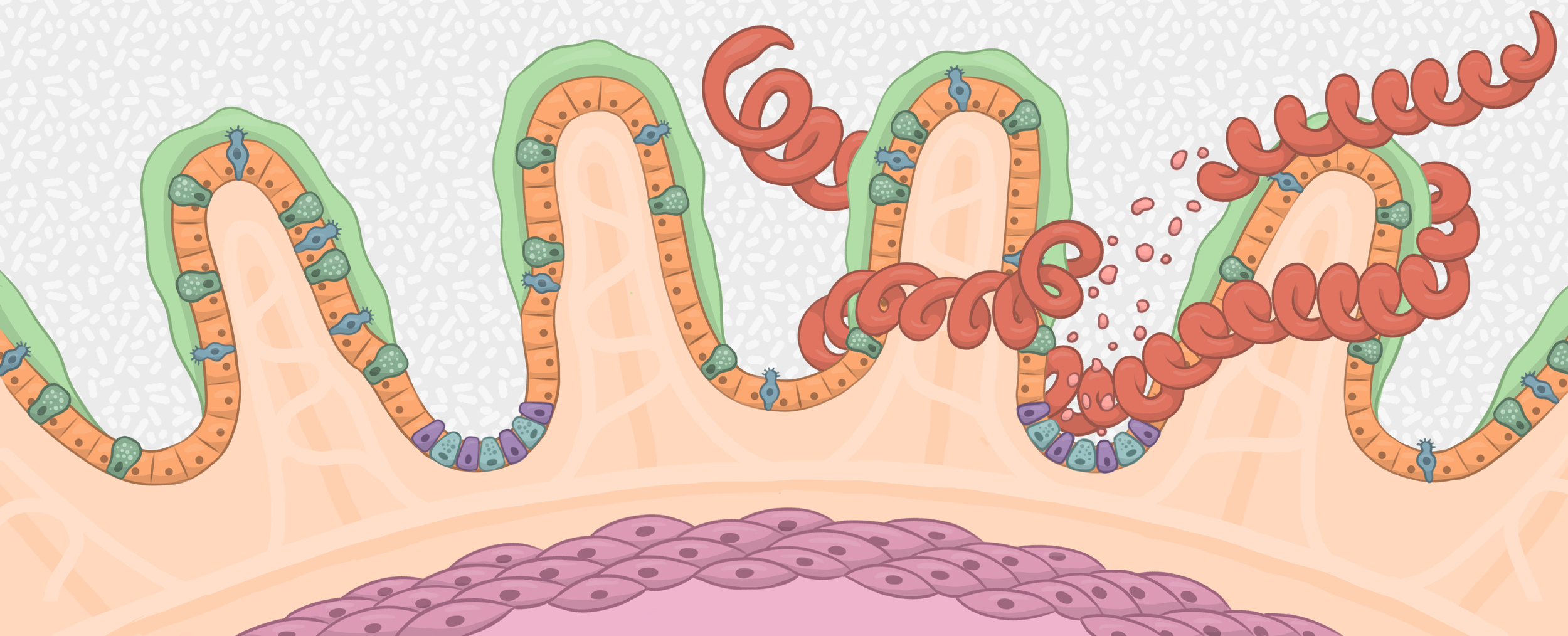
Historically, the immunomodulatory functions of helminths have been attributed to their ability to regulate leukocyte migration, stimulation and effector functions. These discoveries have led to incredible advancements in our understanding of host-parasite interactions and, more broadly, IL4/IL13-driven type 2 immunity. As a consequence, however, understanding how helminths directly impact the function of epithelial cells, a sentinel of host defense at the intestinal barrier, has lagged behind. We have developed an organoid based system that enables the investigation of direct cross-talk between helminths and the intestinal epithelium. In the lab we aim to use this system to identify and investigate helminth-regulated, epithelial-intrinsic pathways that impact host defense during infection.
Helminth regulation of intestinal resilience
One of the most fascinating aspects of enteric helminth infections is the fact that in the face of significant tissue damage, these infections are chronic yet very well tolerated by the host. Distinct from host resistance where the goal is to destroy and eliminate the pathogen, disease tolerance is an active process whereby immune and structural cells restrict tissue damage to maintain host fitness without directly affecting pathogen burden. Our lab investigates how helminths directly promote tissue and barrier integrity to enable their persistence in the intestine. Our work will not only present a paradigm shift in helminth-mediated immune modulation but could also lead to targeted helminth-based therapies aimed at enhancing mucosal healing in diverse settings of intestinal injury and/or inflammation (e.g. inflammatory bowel diseases).